









Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water.
Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water.
Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water. As a liquid, water molecules are bundled close together, making liquid water more dense than gas or solid water.
Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water. As a solid, water molecules form pentagonal or hexagonal lattices, making solid water less dense than liquid water.
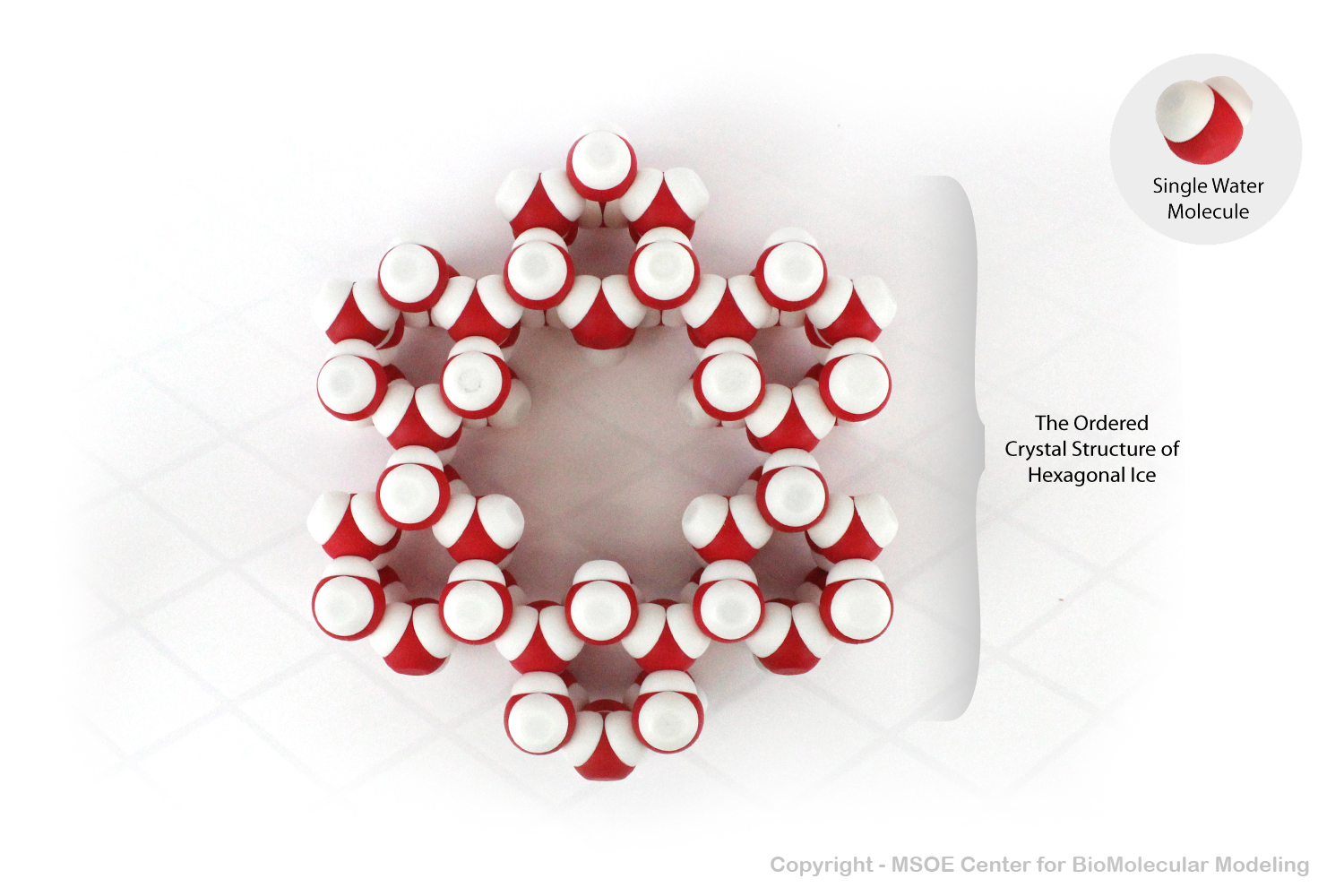
Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water. As a solid, water molecules often form hexagonal lattices, making solid water less dense than liquid water.
Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water. As a solid, water molecules often form hexagonal lattices, making solid water less dense than liquid water.
Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water. As a solid, water molecules often form hexagonal lattices, making solid water less dense than liquid water.
Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water. As a solid, water molecules often form hexagonal lattices, making solid water less dense than liquid water.
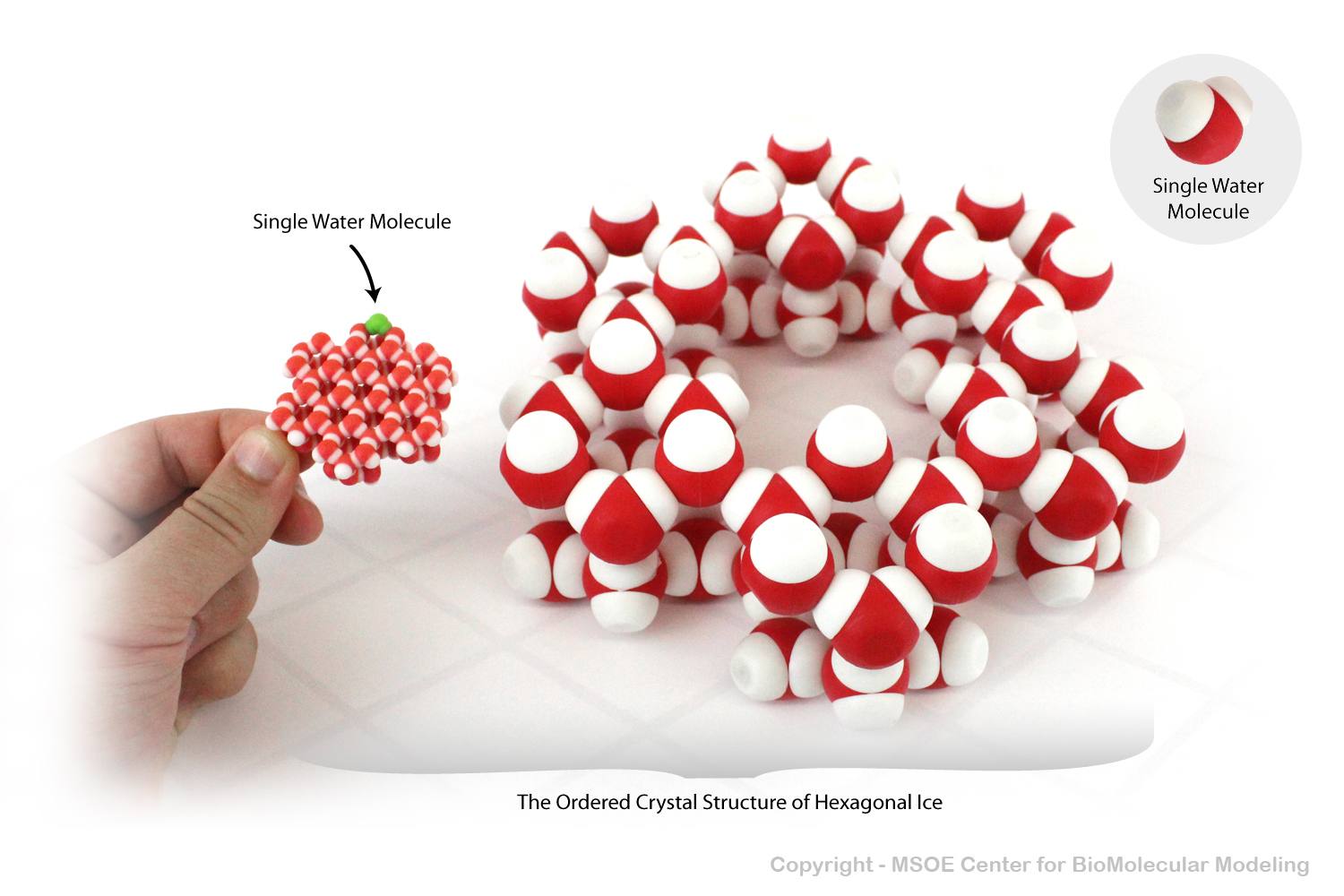
Water molecules demonstrate hydrogen bonding and the polar nature of water. As a solid, water molecules often form hexagonal lattices, making solid water less dense than liquid water.
Water molecules demonstrate hydrogen bonding and the polar nature of water. As a solid, water molecules often form hexagonal lattices, making solid water less dense than liquid water.
Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water. As a solid, water molecules often form hexagonal lattices, making solid water less dense than liquid water.
Note that two different scales are shown in this image. The smaller lattice on the left is approximately one fourth the scale of the larger lattice on the right.
Magnet embedded water molecules demonstrate hydrogen bonding and the polar nature of water. As a gas, water molecules are more spread out and less dense than solid or liquid water. As a liquid, water molecules are bundled close together. As a solid, water molecules often form hexagonal lattices.












