

A protein needs to adopt a final and stable 3-dimensional shape in order to function properly. The Tertiary Structure of a protein is the arrangement of the secondary structures into this final 3-dimensional shape.
The sequence of amino acids in a protein (the primary structure) determines where alpha helices and beta sheets (the secondary structures) occur. These secondary structure motifs then fold into an overall arrangement that is the final 3-dimensional fold of the protein (the tertiary structure).
A summary of primary, secondary and tertiary structure is shown below.

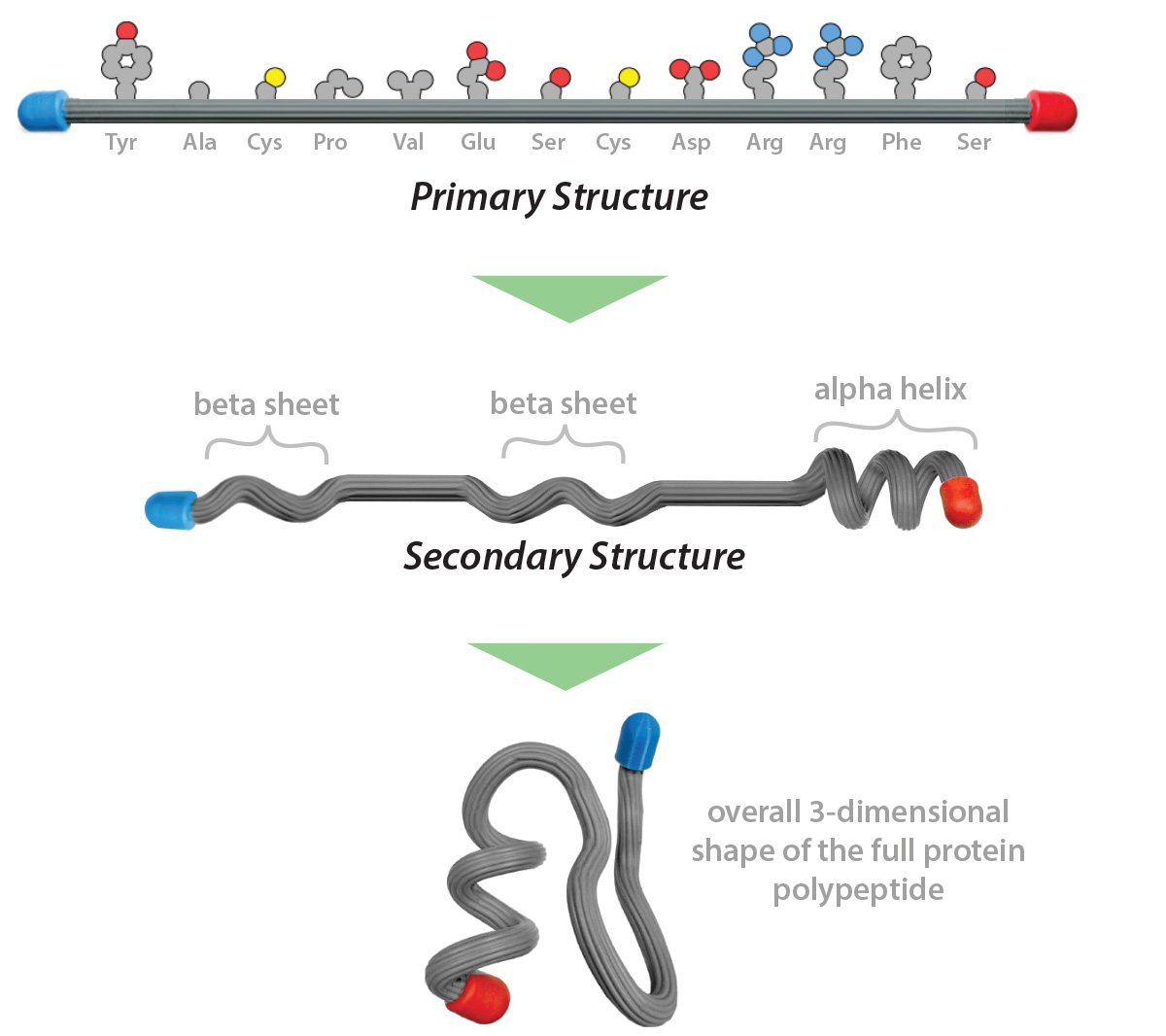
Most proteins fold into their tertiary structure in an aqueous environment - a cell is 60% water. The chemical properties of the various R-groups (sidechains) of the amino acids within the protein chain will influence the way the protein folds in its environment.
Click on the animations below to see how side chains in each of the five categories of amino acids behave when a protein folds.
|
Hydrophobic amino acids will move away from the water and bury themselves in the center of the protein. |
Hydrophilic amino acids will interact with the water molecules, and thus tend to be located on the outer surface of the protein. |
|
Basic (positvely charged) amino acids and Acidic (negatively charged) amino acids create salt bridges, or electrostatic interactions, to further stabilize the tertiary structure. |
Cysteines may form a disulfide bridge, further stabilizing the protein. |
Proteins are amazing molecules because they come in a huge variety of sizes and shapes; each shape suited to perform a specific task. The primary sequence of amino acids in a protein determines its 3-dimensional shape which, in turn, determines how the protein functions.
This structure-function relationship is key to appreciating proteins and protein structure.
The same sequence of amino acids will fold into the same 3-dimensional shape each time it is made, allowing the body to produce millions of identical copies of any specific protein.



© Copyright 1995- - 3D Molecular Designs