

Proteins are some of the most important molecules in biochemistry. They are long strands that fold up into an endless variety of 3-dimensional shapes and sizes, each uniquely structured to perform a specific function in the body.
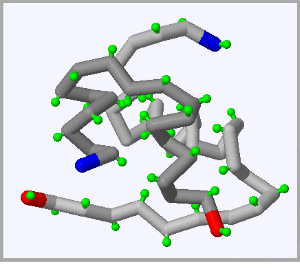
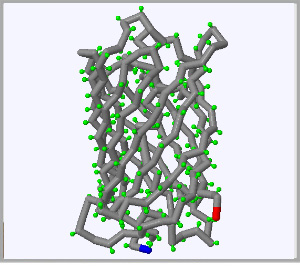
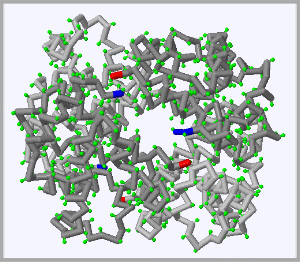
Click on each of the three proteins pictured below to display their structure in the fully interactive display to the right. Click and drag on the display rotate each protein.
The beginning of each protein strand, known as the N-terminus highlighted in blue and the C-terminus, or end of each protein strand is higlighted in red.
|
When you eat sugar, your body senses heightened glucose levels in your blood and releases a protein called Insulin, which helps cells absorb sugar from the blood. |
|
Oceans in the Pacific Northwest often shimmer green as jellyfish rise to the surface. The green color is generated by the Green Fluorescent Protein (GFP) produced by the jellyfish |
|
When you breathe air into your lungs the oxygen binds to a protein called hemoglobin, which carries the normally reactive oxygen safely through the blood and on to all 100 trillion cells in your body. |
Because proteins are such large and complex molecules, scientists often describe their shape as having four aspects of protein structure:

All proteins are made from small molecules called amino acids that are joined together like links in a chain. There are 20 different types of amino acids that can be linked together in various orders and frequencies.
Each type of protein has a unique sequence of amino acids. The specific order of amino acids in a protein chain is referred to as the protein's Primary Structure
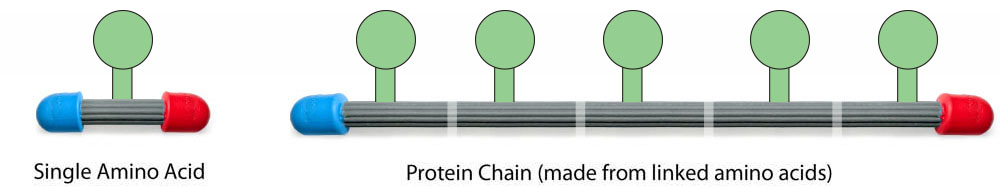

The chemical structure of each of the twenty types of amino acids are identical in some ways, but unique in others.
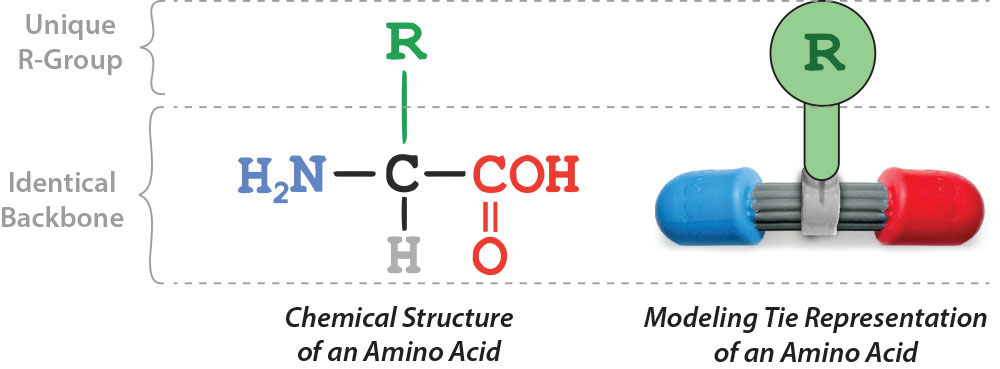

Shown below is the chemical structure of a single amino acid (left) and a physical model of a single amino acid (right).
Use the buttons below to highlight the different parts of a single amino acid and display each part in the interactive 3-dimensional window (far right).


Let's take a closer look at the unique part of the 20 different amino acids. The R-group, also called the side chain, is considered the functional group of each amino acid. The R-group gives each amino acid its unique attributes and sorts each amino acid into five categories based on these unique attributes.
| Hydrophobic - water-avoiding amino acids with m primarily non-polar carbon atoms in their R-group. |
| Hydrophillic - water-loving amino acids with some polar oxygen and nitrogen atoms in their R-group. |
| Positively Charged - water-loving amino acids with a net positive charge (+) due to some nitrogen, but no oxygen atoms in their R-group. |
| Negatively Charged - water-loving amino acids with a net negative charge (-) due some oxygen, but no nitrogen atoms in their R-group. |
| Cysteine - a unique amino acid that can form strong bonds (called disulfide bonds) with other cysteine amino acids. |

Click on the table below to explore the shapes of all twenty amino acids and their R-groups. Click Here to download a chart summarizing all 20 amino acids and their R-group shapes.
 Alanine Alanine |
 Arginine Arginine |
 Asparagine Asparagine |
 Aspartic Acid Aspartic Acid |
 Cysteine Cysteine |
 Glutamine Glutamine |
 Glutamic Acid Glutamic Acid |
 Glycine Glycine |
 Histidine Histidine |
 Isoleucine Isoleucine |
 Leucine Leucine |
 Lysine Lysine |
 Methionine Methionine |
 Phenylalanine Phenylalanine |
 Proline Proline |
 Serine Serine |
 Threonine Threonine |
 Tryptophan Tryptophan |
 Tyrosine Tyrosine |
 Valine Valine |
Amino acids are chemically linked together to make long amino acid chains through a condensation reaction (sometimes called dehydration synthesis).
This chemical reaction will form a bond linking the backbone regions of two amino acids together. This type of bond between two amino acids is called a peptide bond.
 This reaction also creates a molecule of water as a byproduct. One oxygen atom and one hydrogen atom come from the carboxylic acid group of the first amino acid and join with another hydrogen atom from the amino group of the second amino acid.
This reaction also creates a molecule of water as a byproduct. One oxygen atom and one hydrogen atom come from the carboxylic acid group of the first amino acid and join with another hydrogen atom from the amino group of the second amino acid.
Click Here to display a dipeptide connected by a peptide bond (show in yellow) in the interactive display to the right.

A chain of many amino acids linked together by peptide bonds is called a polypeptide or a protein chain.
The specific sequence of amino acids in a polypeptide is known as the protein's primary structure. The twenty types of amino acids can be joined together in any order or frequency, allowing for an astronomical variety of potential primary structures.
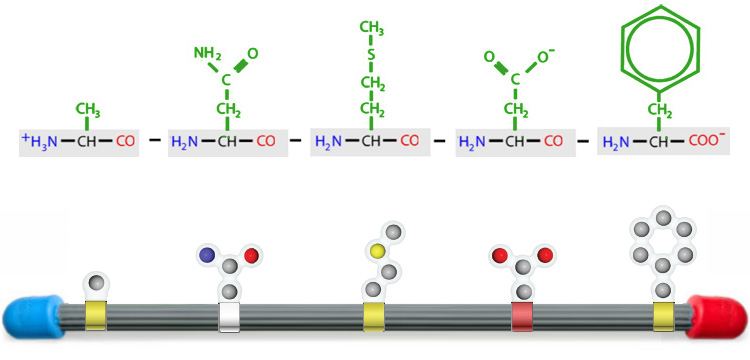
Each type of protein in our body has a unique primary structure. This specific order of amino acids determines the protein's final 3-dimensional shape and function.



© Copyright 1995- - 3D Molecular Designs