
Catalase is an enzyme that has evolved to protect us from molecules containing reactive oxygen such as hydrogen peroxide. It binds hydrogen peroxide (a substrate) to its active site, and converts it to water and molecular oxygen (O2). O2 is a gas that forms the bubbles students see when they do the catalase lab in their biology class.



Catalase is a 497 amino acid long protein that folds up into a complex 3D structure composed of alpha helices and beta sheets, connected by loops of protein structure.


Catalase uses an iron atom (Fe) to create the chemistry that cleaves hydrogen peroxide into water and O2. This iron atom is the central atom of an enzyme cofactor known as a heme group.
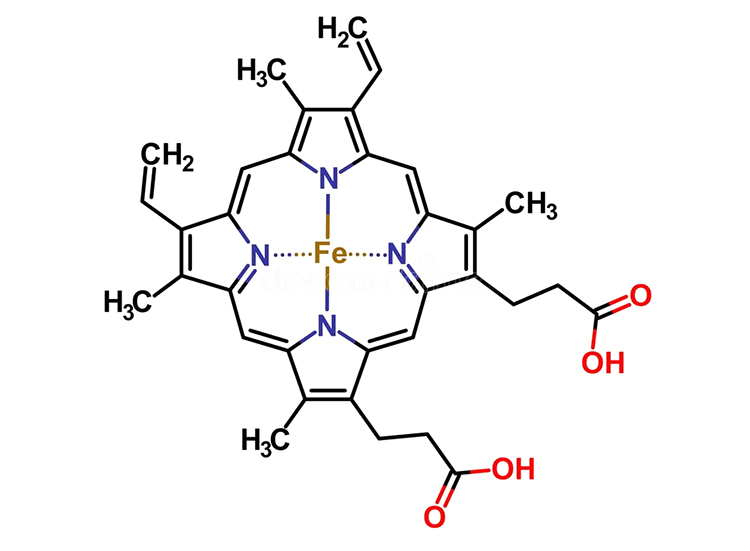


This heme group is buried deep in the center of the catalase enzyme. Why might a change in pH effect the activity of catalase?


Perhaps it has something to do with the amino acid sidechains of the catalase protein that are either negatively charged acidic amino acids or positively charged basic amino acids.
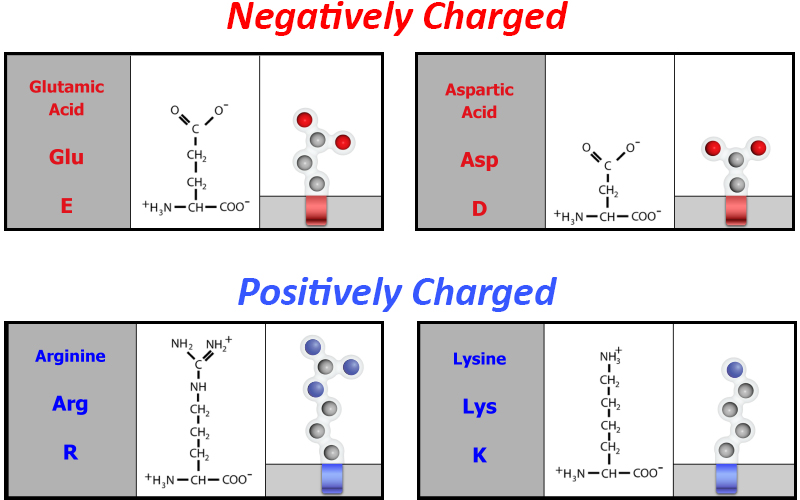

Explore the Jmol image to the right. Can you find examples of electrostatic interactions between acidic and basic amino acid sidechains? Can you find an example of an electrostatic interaction that stabilizes the heme group in the center of the protein?


One example of electrostatic interactions between acidic and basic amino acid sidechains is arginine 156 and glutamic acid 105.


One example of electrostatic interactions between basic amino acid sidechains and the positive region of the heme group is arginine 344.


What happens to catalase activity when it is heated? Let's color areas of the catalase structure that are less stable in red, which are prone to denaturation upon heating.
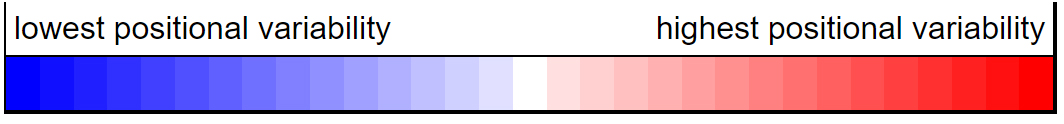


Are you surprised to see that the N-terminal end of the enzyme, highlighted in green, appers to be very stable? Why might this be?


We are currently looking at one chain of the catalase protein. But the full protein is comprised of four identical chains! The n-terminal domain of each chain is buried in the other three chains, making it quite stable.


Let's also explore how the hydrophobic sidechains, highlighted in yellow, are distributed. Use the slider below to view the interior of the catalase structure. What sort of interactions stabilize the heme group in the center of the catalase structure?
